To know more about reverse osmosis (RO), which is the key process to produce drinking water, one needs to know about the basis osmosis process first.
Osmosis Process
Osmosis is a phenomenon where pure water flows from a dilute solution through a semi permeable membrane to a higher concentrated solution. Semi permeable means that the membrane will allow small molecules and ions to pass through it but acts as a barrier to larger molecules or dissolved substances. To illustrate this, assume that a semi permeable membrane is placed between two compartments in a tank. Assume the membrane is permeable to water, but not to salt. If we place a salt solution in one compartment and pure water solution in the other one, the system will try to reach equilibrium by having the same concentration on both sides of the membrane. The only possible way to do this is for water to pass from the pure water compartment to the saltwater compartment.
As water passes through the membrane to the salt solution, the level of liquid in the saltwater compartment will rise until enough pressure, caused by the difference in levels between the two compartments, is generated to stop the osmosis. This pressure, equivalent to a force that the osmosis seems to exert in trying to equalize concentrations on both sides of the membrane, is called osmotic pressure.
Reverse Osmosis
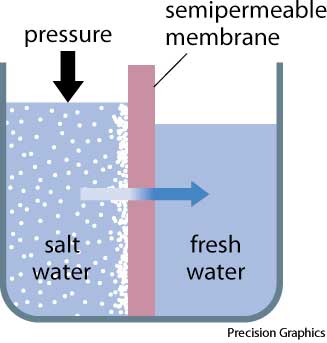
If pressure greater than the osmotic pressure is applied to the high concentration the direction of water flow through the membrane can be reversed. This is called reverse osmosis (abbreviated RO). Note that this reversed flow produces pure water from the salt solution, since the membrane is not permeable to salt.
How Does Reverse Osmosis Work?
Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration. Osmosis is a special case of diffusion in which the molecules are water and the concentration gradient occurs across a semipermeable membrane. The semipermeable membrane allows the passage of water, but not ions (e.g., Na+, Ca2+, Cl-) or larger molecules (e.g., glucose, urea, bacteria). Diffusion and osmosis are thermodynamically favorable and will continue until equilibrium is reached. Osmosis can be slowed, stopped, or even reversed if sufficient pressure is applied to the membrane from the ‘concentrated’ side of the membrane.
Reverse osmosis occurs when the water is moved across the membrane against the concentration gradient, from lower concentration to higher concentration. To illustrate, imagine a semipermeable membrane with fresh water on one side and a concentrated aqueous solution on the other side. If normal osmosis takes place, the fresh water will cross the membrane to dilute the concentrated solution. In reverse osmosis, pressure is exerted on the side with the concentrated solution to force the water molecules across the membrane to the fresh water side.
Membrane Separation Technologies
If pressure greater than the osmotic pressure is applied to the high concentration the direction of water flow through the membrane can be reversed. This is called reverse osmosis (abbreviated RO). Note that this reversed flow produces pure water from the salt solution, since the membrane is not permeable to salt.